GLYXAMBI® contains Empagliflozin and Linagliptin that has 3 PIVOTAL AND CONVINCING Cardiovascular Outcome Trials (CVOTs).1



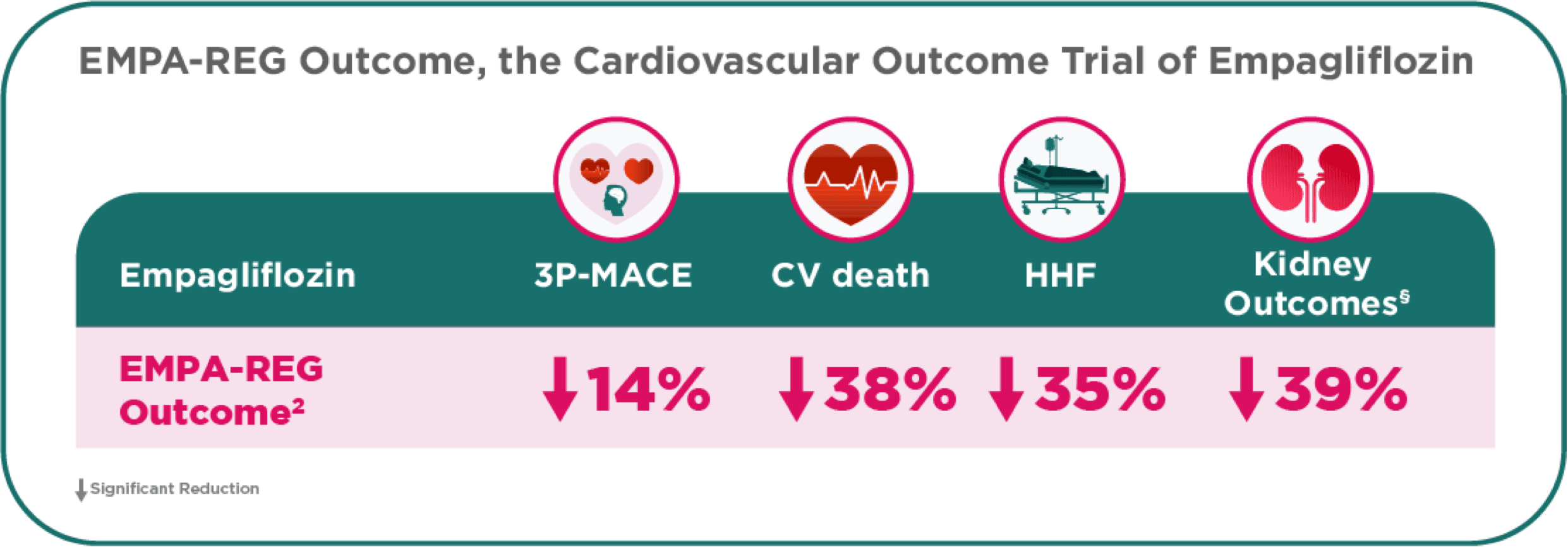
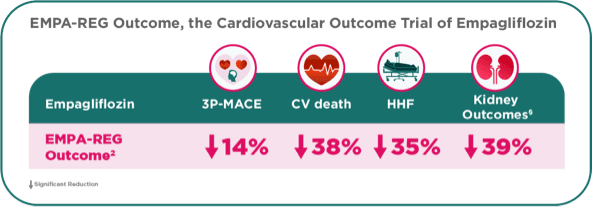
Empagliflozin in the EMPA-REG OUTCOME demonstrated Cardio-Renal Benefits and UNIQUELY demonstrated a significant 38% relative risk reduction in CV death.2,5
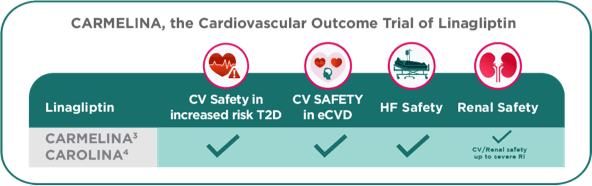
Linagliptin in the CARMELINA and CAROLINA Trials demonstrated Cardio-Renal Safety.6
- †EMPA-REG OUTCOME® trial: Primary outcome was reduction in CV events defined as composite endpoint of CV death, non-fatal MI, or non-fatal stroke. In patients with T2D and established CV disease, on top of standard of care.2
- §Hard kidney outcomes (excluding albuminuria and including eGFR decline) with different definitions for substantial loss of kidney function.
References
- Glyxambi® Singapore Prescribing Information.
- Zinman B, Wanner C, Lachin J, et al. EMPA-REG OUTCOME® Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128.
- Rosenstock J, Perkovic V, Johansen OE, et al; for the CARMELINA Investigators. E_ect of linagliptin vs. placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk. JAMA. 2019;321(1):69-79.
- Rosenstock J, Kahn SE, Johansen OE. E_ect of linagliptin vs. glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019;322(12):1155-1166.
- Wanner et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016 Jul 28;375(4):323-334.
- Trajenta® Singapore Prescribing Information.